【文献名】
Adriana Buitrago-Lopez et al: Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ 2011; 343:d4488
【要約】
<Objective>
To evaluate the association of chocolate consumption with the risk of developing cardiometabolic disorders.
<Design>
Systematic review and meta-analysis of randomised controlled trials and observational studies.
<Data sources>
Medline, Embase, Cochrane Library, PubMed, CINAHL, IPA, Web of Science, Scopus, Pascal, reference lists of relevant studies to October 2010, and email contact with authors.
<Study selection>
Randomised trials and cohort, case-control, and cross sectional studies carried out in human adults, in which the association between chocolate consumption and the risk of outcomes related to cardiometabolic disorders were reported.
<Data extraction>
Data were extracted by two independent investigators, and a consensus was reached with the involvement of a third. The primary outcome was cardiometabolic disorders, including cardiovascular disease (coronary heart disease and stroke), diabetes, and metabolic syndrome. A meta-analysis assessed the risk of developing cardiometabolic disorders by comparing the highest and lowest level of chocolate consumption.
<Results>
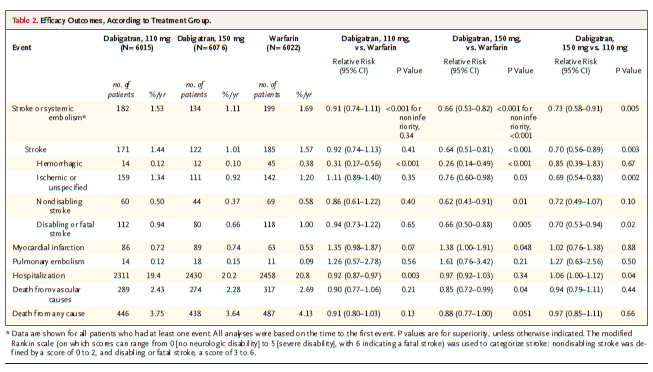
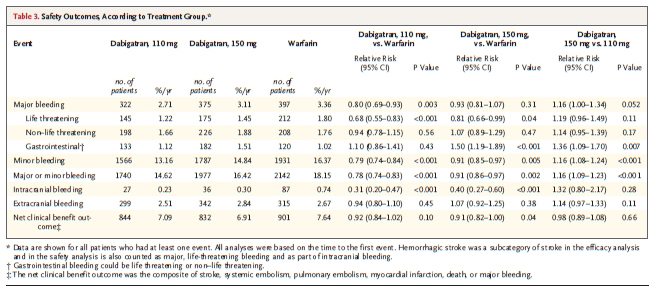
From 4576 references seven studies met the inclusion criteria (including 114 009 participants). None of the studies was a randomized trial, six were cohort studies, and one a cross sectional study. Large variation was observed between these seven studies for measurement of chocolate consumption, methods, and outcomes evaluated. Five of the seven studies reported a beneficial association between higher levels of chocolate consumption and the risk of cardiometabolic disorders. The highest levels of chocolate consumption were associated with a 37% reduction in cardiovascular disease (relative risk 0.63 (95% confidence interval 0.44 to 0.90)) and a 29% reduction in stroke compared with the lowest levels.
<Conclusions>
Based on observational evidence, levels of chocolate consumption seem to be associated with a substantial reduction in the risk of cardiometabolic disorders. Further experimental studies are required to confirm a potentially beneficial effect of chocolate consumption.
【開催日】
2011年9月7日